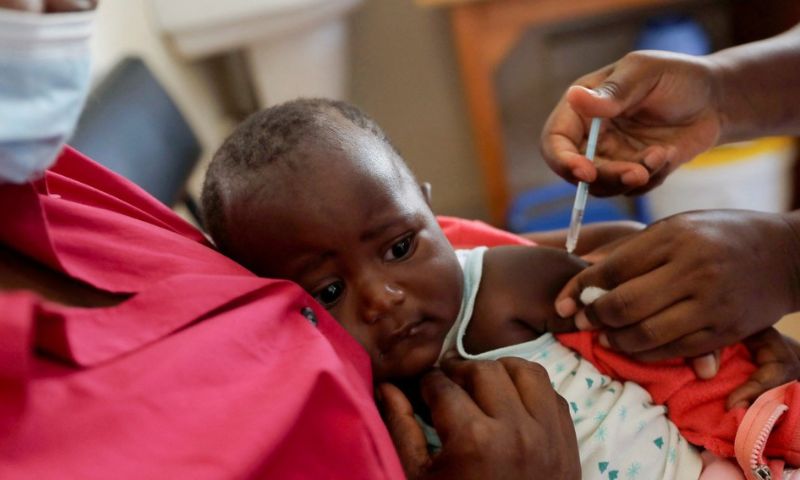
GENEVA: The World Health Organization (WHO) announced on Monday that its experts have recommended the approval of a second malaria vaccine for children, R21/Matrix-M, developed by Britain’s Oxford University. The vaccine, manufactured by the Serum Institute of India, has already been approved for use in Burkina Faso, Ghana, and Nigeria.
WHO Chief Tedros Adhanom Ghebreyesus expressed his enthusiasm, stating, “As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two.”
In 2021, the RTS,S vaccine, produced by British pharmaceutical giant GSK, became the first malaria vaccine to be recommended by the WHO for preventing malaria in children residing in areas with moderate to high malaria transmission.
Significance of New Malaria Vaccine
Dr. Matshidiso Moeti, the WHO’s Regional Director for Africa, highlighted the significance of the new vaccine for the continent. She emphasized its potential to address the substantial demand-and-supply gap in malaria prevention efforts. Dr. Moeti stated, “Delivered at measure and widely rolled out, the two vaccines can help improve malaria prevention and control efforts and save hundreds of thousands of young lives in Africa from this deadly disease.”
The approval of the R21/Matrix-M vaccine represents a significant milestone in the global fight against malaria, offering a promising tool to protect vulnerable populations, particularly children, from this life-threatening disease.